The FDA’s Decision to Ban Compounds: What You Need to Know
The FDA’s decision to ban compounded semaglutide stems from concerns over safety, efficacy, and quality control. Compounded versions lack the rigorous testing and oversight of FDA-approved medications like Ozempic and Wegovy. Rafael Olartecoechea, founder of Vive Ageless Weight Loss Center, offers personalized weight loss treatments, including FDA-approved medications, to ensure patient safety and effectiveness. For more information, contact us or book an appointment right now. We have convenient locations in Coral Gables FL, and Pinecrest FL.
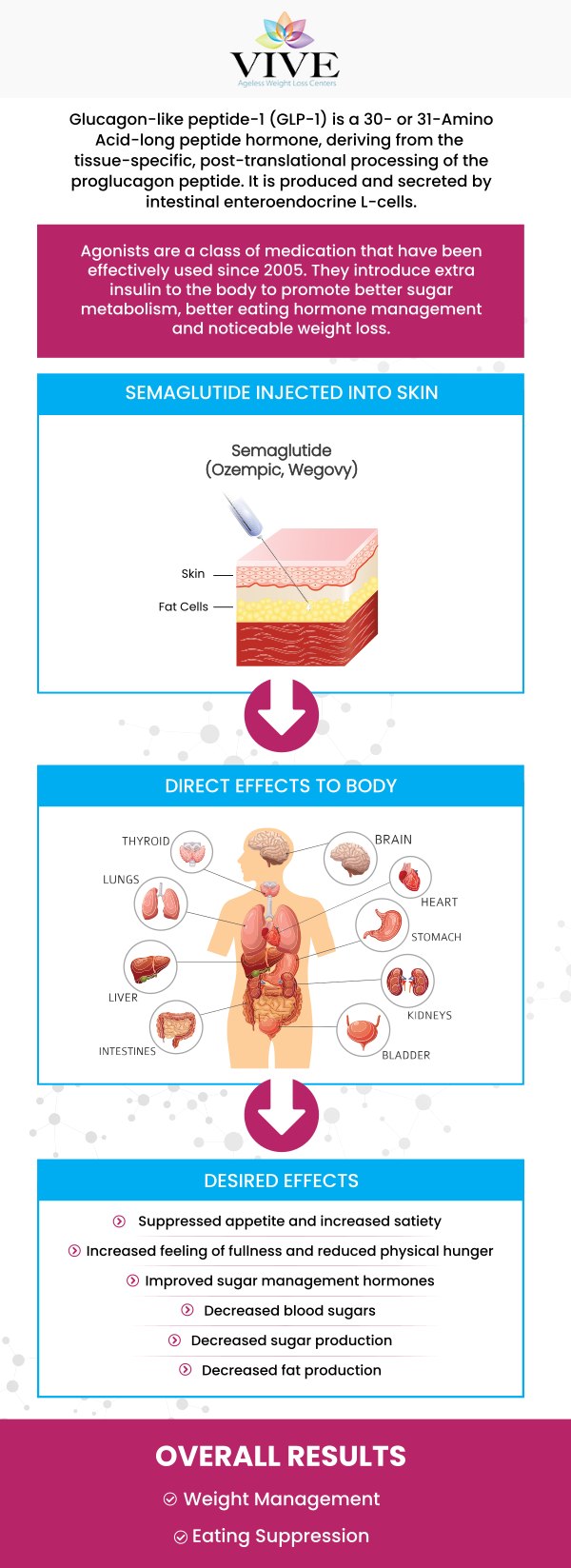
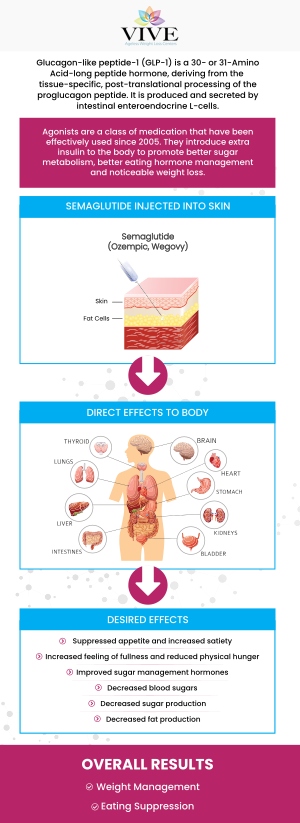


Table of Contents:
What is the difference between semaglutide GLP-1 and compounded semaglutide?
What is the FDA’s decision to ban compounded medications about?
How does the ban on compounded semaglutide affect patients?
The primary difference between semaglutide GLP-1 and compounded semaglutide lies in their formulation, regulation, and the way they are prepared. Semaglutide GLP-1, the active ingredient found in various brand-name medications such as Ozempic and Wegovy, is a commercially produced medication. Such medications undergo rigorous testing and clinical trials to establish their safety and efficacy. The dosing and formulation of commercially made medications are consistent and determined by the manufacturer.
On the other hand, compounded semaglutide GLP-1 is not commercially produced but rather prepared by pharmacists on an individual basis. The formulation and dosage can be customized to meet the specific needs of each patient. However, compounded medications are not FDA-approved, as their safety and efficacy are not subject to the same level of regulatory scrutiny. The responsibility for ensuring the quality and safety of the medication lies in the hands of the compounding pharmacist.
To summarize, semaglutide GLP-1-based medications, such as Wegovy, are an FDA-approved treatment with established safety and efficacy profiles. Compounded semaglutide GLP-1, on the other hand, offers customization but lacks the high level of regulatory oversight and consistency associated with brand-name formulations.
The FDA’s decision to ban compounded medications is a regulatory action aimed at addressing safety concerns associated with drugs that are made by compounding pharmacies. Compounded medications are custom-prepared by pharmacies to meet the unique needs of individual patients. However, these drugs are not subject to the same rigorous testing and approval process as FDA-approved medications, which raises significant concerns about their safety, effectiveness, and quality.
The FDA has expressed particular concerns about compounded medications because they often lack the necessary oversight that ensures consistent quality. In many cases, compounded drugs may contain harmful ingredients, incorrect dosages, or impurities that can lead to adverse health effects. The decision to ban compounded medications comes after the FDA identified a growing number of unapproved compounded drugs in the market, including versions of FDA-approved medications like semaglutide, which are being produced without proper regulatory oversight.
This decision impacts various drugs, particularly in the context of weight loss treatments like semaglutide, where compounded versions are being sold despite the availability of FDA-approved alternatives. The FDA’s ban seeks to protect public health by ensuring that only regulated, tested, and approved medications are available to patients. Healthcare providers will need to switch to FDA-approved options to ensure patient safety and efficacy in treatment.
The FDA’s decision to ban compounded semaglutide significantly impacts patients who have relied on these unapproved versions for weight loss or diabetes management. Compounded semaglutide was often marketed as a more affordable alternative to FDA-approved medications like Ozempic and Wegovy. However, these compounded versions have been associated with serious health risks, including dosing errors, adverse reactions, and the presence of harmful impurities.
With the ban in place, patients may face challenges in accessing affordable treatment options. Insurance coverage for FDA-approved medications may be limited, especially for off-label uses such as weight loss in individuals without obesity. This could lead to increased out-of-pocket expenses for patients seeking effective treatment. Additionally, the discontinuation of compounded semaglutide may disrupt ongoing treatment plans for those who have experienced positive outcomes with these medications.
Patients affected by the ban should consult with their healthcare providers to discuss alternative treatment options and explore potential financial assistance programs to mitigate the impact of the ban on their access to necessary medications. Our clinics are located at two locations in Coral Gables, FL, and Pinecrest, FL. You can contact us or book an online appointment for both locations. We serve clients from Coral Gables FL, Miami FL, Pinecrest FL, South Miami FL, Coconut Grove FL, Brickell FL, Kendall FL, Richmond Heights FL, and Westchester FL.


Additional Services You May Need
▸ Obesity
▸ Peptide Therapy
▸ Anti-Aging Medicine
▸ Medical Weight Loss
▸ Meal Planning
▸ Nutritional Consultations
▸ Metabolic Insufficiency Treatment
▸ Hormone Imbalance
▸ Inactive Lifestyle
▸ Boost Metabolism
▸ Food Sensitivity/Allergies
▸ Diabetic Weight Loss
▸ Body Mass Index (BMI)
▸ Body Fat Percentage









